
In fact, this reaction is so iconic that we define a base as any compound that undergoes neutralisation reaction with acids.ĭuring neutralisation, acids react with bases to form salt and water only. It is termed neutralisation as the products are salt and water only, which usually gives a pH of around 7. Turning acids into water: neutralisationĪnother way of neutralising the danger of acids is to neutralise acids with bases. Instead, they added sodium carbonates to react with the hydrochloric acid, turning it into relatively harmless salt, water, and carbon dioxide gas. They cleaned up the acid, but not by sweeping it away. In 2019 the Singapore Civil Defence Force conducted a drill simulating the spillage of concentrated hydrochloric acid.

This poses a considerable risk of an accidental spill.ĢHCl (aq) + Na 2CO 3 (s) ⟶ 2NaCl (aq) + CO 2 (g) + H 2O (l) To meet the demand, we import large amounts of concentrated sulfuric acid and hydrochloric acid from Malaysia via the Tuas Second Link.

Singapore has a big chemical industry that requires acid as one of the many raw materials. However, we are not completely spared from the environmental danger of strong acid. This salt is made up of calcium cation from the carbonate, and nitrate anion from the acid.īesides calcium carbonate, all carbonates react with acids to give the same trio of products: a salt, carbon dioxide gas, and water. The anion of the acid will become the anion of the salt, while the cation of the carbonate will be the cation of the salt.ĢH NO 3 (aq) + CaCO 3 (s) ⟶ Ca( NO 3) 2 (aq) + CO 2 (g) + H 2O (l)įor example, nitric acid reacts with calcium carbonate to form calcium nitrate salt, carbon dioxide gas, and water. Metal reactions with water: Cold water reacts with potassium, sodium, and calcium. In the reactivity series, zinc is above copper, implying that zinc is more reactive than copper. A reactivity series is typically a vertically presented model with the most-reactive element placed at the top of the series and the least-reactive element placed at the bottom. Like in the acid-metal reaction, the exact chemical composition of the salt depends on the type of acid and carbonate used. The reactivity series is a collection of metals arranged in decreasing order of reactivity. The reactivity series is a series of metal elements, and sometimes carbon and hydrogen, that is arranged according to their reactivity. This forms water and carbon dioxide gas, turning the monument into thin air rather literally.Ī salt is also produced as a by-product. The hydrogen ions of acid react with the carbonate ions of calcium carbonate. And also the carbonatesīesides corroding metallic structures, acid rain also gnaws away stone monuments made up of limestone, which is essentially calcium carbonate. They have a greater propensity to lose electrons to the hydrogen ions of acid during the metal-acid reaction.Īcids react with reactive metals to form a salt and hydrogen gas.
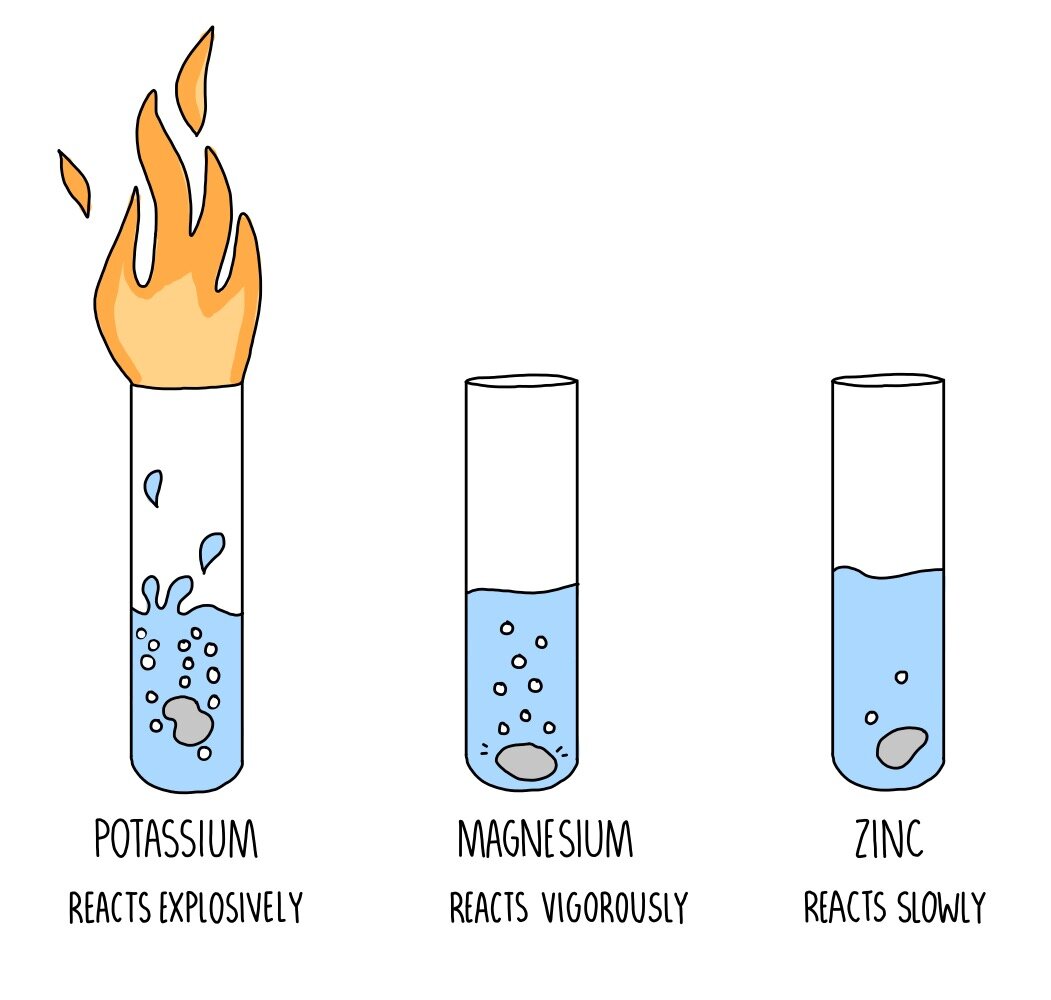
As we move up the series, the metals above are more reactive. To help us identify what react, we can refer to the reactivity series. While some react violently with acids, others like copper, silver, platinum, and gold are so stable that they do not react. However, metals have different reactivity. Winning is represented by having the oxide.In the reactivity series of metals, only lead and the metals above can react with acids. The top team always beats the lower teams (there are no shock results!). Think of it like the football league tables. If you are told that Blobbium is below iron in the reactivity series, then B is right. If you are told that Blobbium is above iron in the reactivity series, then A is right. It looks OK but what about this one? blobbium oxide + iron gives blobbium + iron oxide (B)īoth COULD be OK but which one really happens? You cannot tell until you know the position of blobbium in the reactivity series.

The Reactivity Series helps us to predict the outcome of some reactions.ĭoes this reaction proceed? blobbium + iron oxide gives blobbium oxide + iron (A) We can also add in some non-metals (hydrogen goes between iron and copper carbon goes between magnesium and iron). We can add in other metals but this is good enough for now. The reactivity series shows the list of metals starting with the most reactive at the top and the least reactive at the bottom.


 0 kommentar(er)
0 kommentar(er)
